The ozone layer is the layer with the highest levels of ozone (O3) in the Earth’s atmosphere. This layer absorbs 93-99% of the ultraviolet rays emitted by the sun, which are harmful to terrestrial organisms: ultraviolet rays. About 91% of the ozone in the Earth’s atmosphere is found in this region. It is located at the bottom of the stratosphere, approximately 10 to 50 km above ground level.
1913 Its presence was discovered by the French physicists Charles Fabry and Henry Bison. The British GMB. Dobson learned about its structure and properties, and developed a spectrometer that could measure ozone in the stratosphere. Between 1928 and 1958 he established a worldwide network of ozone monitoring stations. It’s still working. In his memory, the total amount of ozone in the atmosphere above his head is called the Dobson unit. September 16 is Ozone Day.
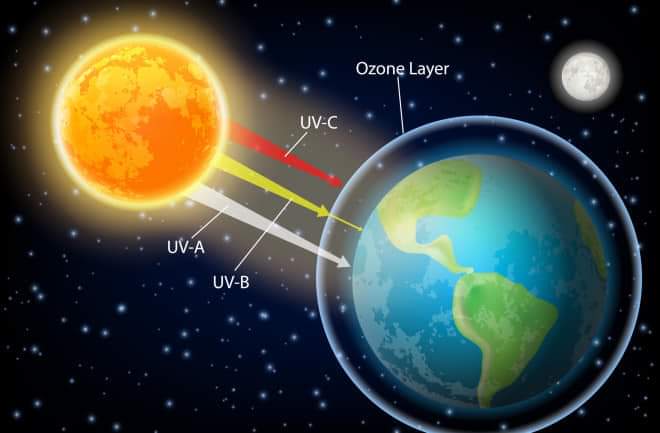
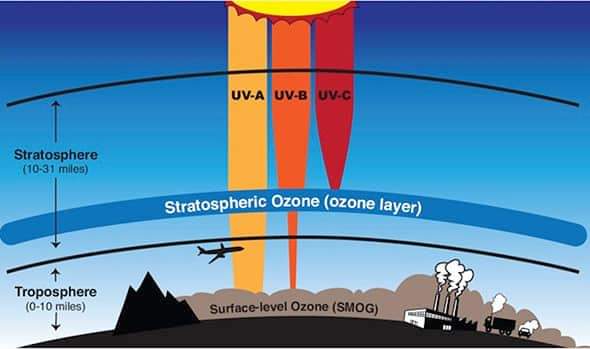
Sunlight layers are divided into three types — UV-A, UV-B and UV-C. UV-A has a wavelength of 315 to 400 nanometers (nanometers). UV-B is 280 nm to 315 nm and UV-C is 100 nm to 280 nm. When UV-C enters these oxygen molecules, they decompose and split into two oxygen atoms. But the atom of most elements in air cannot exist alone. In the case of oxygen, it combines with another molecule to form the ozone molecule. This process was invented by the mathematician Sydney Chapman (1888-1970).
Ozone is formed in other ways near the Earth’s surface. Lightning is one of the causes. Lightning is an electric spark. Ozone also produces other electric sparks. Electric motors are an example. Larger motors used in lifts and the like produce more ozone. In addition to motors, ozone-producing machines use high voltage to operate photocopiers, television sets, and laser printers. Ozone was discovered by an electric machine.

In addition, some other human activities cause ozone depletion. Ozone is formed when oxides of nitrogen, carbon monoxide, and some volatile organic matter, such as methane, react in the presence of sunlight. These chemicals are mainly found in urban areas. However, they can reach miles away. This resulting ozone can cause air pollution known as “photochemical smog”. Ozone is also a greenhouse gas. Breathing air that contains more ozone can cause lung diseases. People with asthma are more likely to have it. It can cause problems with the blood vessels and even the heart.
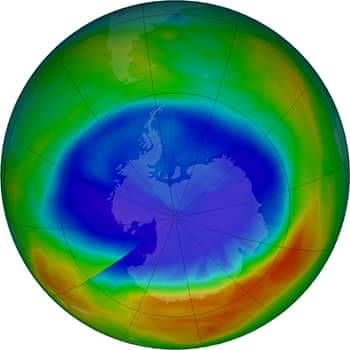
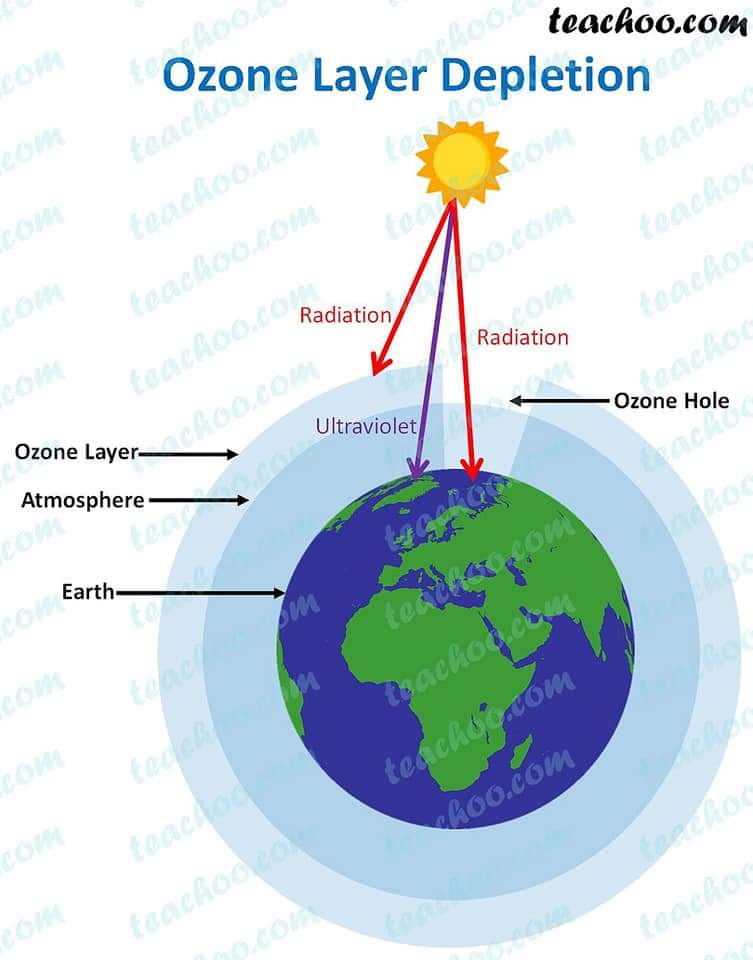
Depletion of the ozone layer has now become a major human hazard. Nitrous oxide (NO), nitric oxide (N2O), hydroxyl (OH), atomic chlorine (Cl) and bromine (Br) cause damage to the ozone layer. Man-made chlorofluorocarbons (CFCs) and bromofluorocarbons are the biggest threat to ozone today. Hydrochlorofluorocarbons have recently been found to cause ozone depletion.



Recent Comments