
In our daily lives, we believe we touch objects and surfaces, but fundamentally, this isn’t accurate. All matter is composed of atoms, which include electrons. When atoms come close, their electrons repel each other. This fundamental principle of physics means that objects never actually touch. What we perceive as touch is the electromagnetic force produced by the repulsion of electrons.
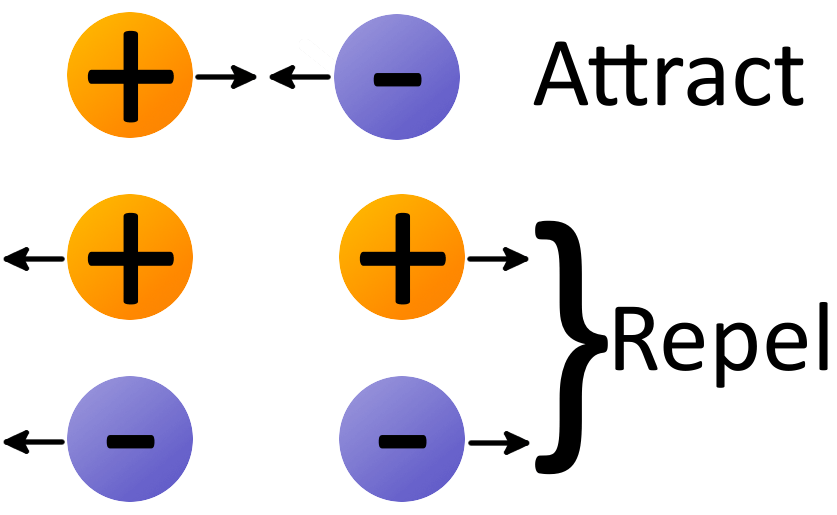
To elaborate, every object is made up of atoms, and atoms contain electrons. Electrons have a neutral charge, but when they come close to each other, they repel. This electron repulsion generates an electromagnetic force that our brain interprets as touch. This interaction happens at a minuscule distance, approximately 10^-8 meters. So, when two particles “touch,” what actually occurs is an atomic reaction, driven by the repulsion of their electrons.
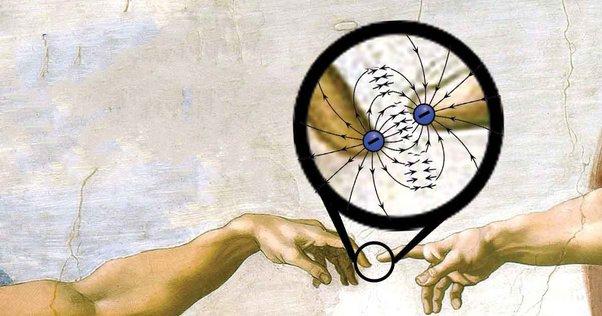
In essence, our perception of touch is a result of these tiny forces acting at a microscopic level. Understanding this concept reshapes our view of the physical interactions around us, revealing the intricate and fascinating nature of the atomic world.






Recent Comments