
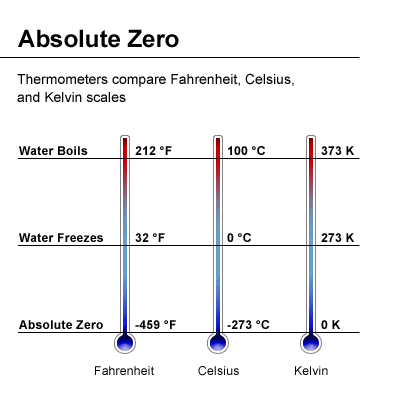
Absolute zero is the lowest possible temperature in the universe, where no heat is present. This is a state where all molecular motion ceases entirely. In simpler terms, when there is no movement among the molecules, there is no heat, and this is what we call absolute zero.
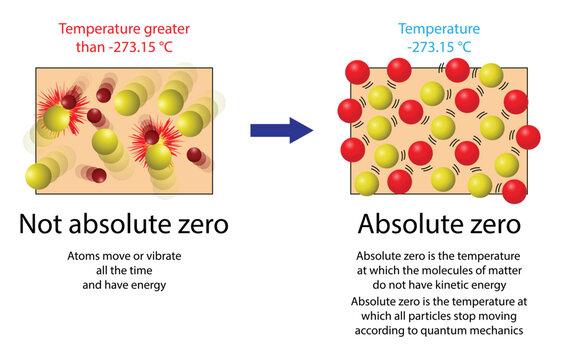
Heat is the result of molecules moving and colliding with each other. The more these molecules move, the higher the temperature rises. Conversely, when we cool something down, we are slowing down the movement of its molecules.
As the movement decreases, the temperature drops. When molecular motion is reduced to its minimum, we reach the point of absolute zero.
To better understand this, imagine applying brakes to a moving car or bike. As you press the brakes, the vehicle slows down. However, once the vehicle comes to a complete stop, pressing the brakes further does not make any difference. Similarly, at absolute zero, since all molecular motion has ceased, no further cooling or changes can occur.




Recent Comments