
The spin of an electron is 1/2. The spin is 1/2 for the proton. A hydrogen atom consists of a proton and an electron. Then there are two possibilities for total spin. One or both can be in the same direction. Then you get 1 total spin. Or in the opposite direction. Then we get 0.
There is a slight difference in energy level between these two levels. We know that the energy at n = 1 is -13.6 eV. F (i.e. total spin) 1 is slightly greater than this. Decrease when 0. In any case there is a slight difference between these two energy levels. This is called hyperfine splitting.
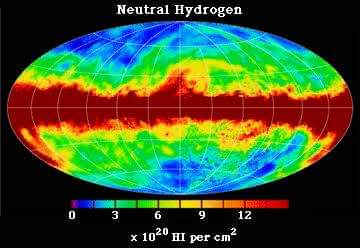
The photon is emitted as it descends from the upper level. The wavelength of that photon is 21.1 cm. Approximately 140MHz frequency. The lifetime of this transition is approximately 11 billion years. Therefore it is very difficult to monitor it in the lab.
But it can be observed elsewhere. That is Hydrogen atomic clouds. These clouds contain a lot of hydrogen atoms. Therefore, we can observe this radiation from these. This radiation is used to study atomic hydrogen clouds.





Recent Comments