
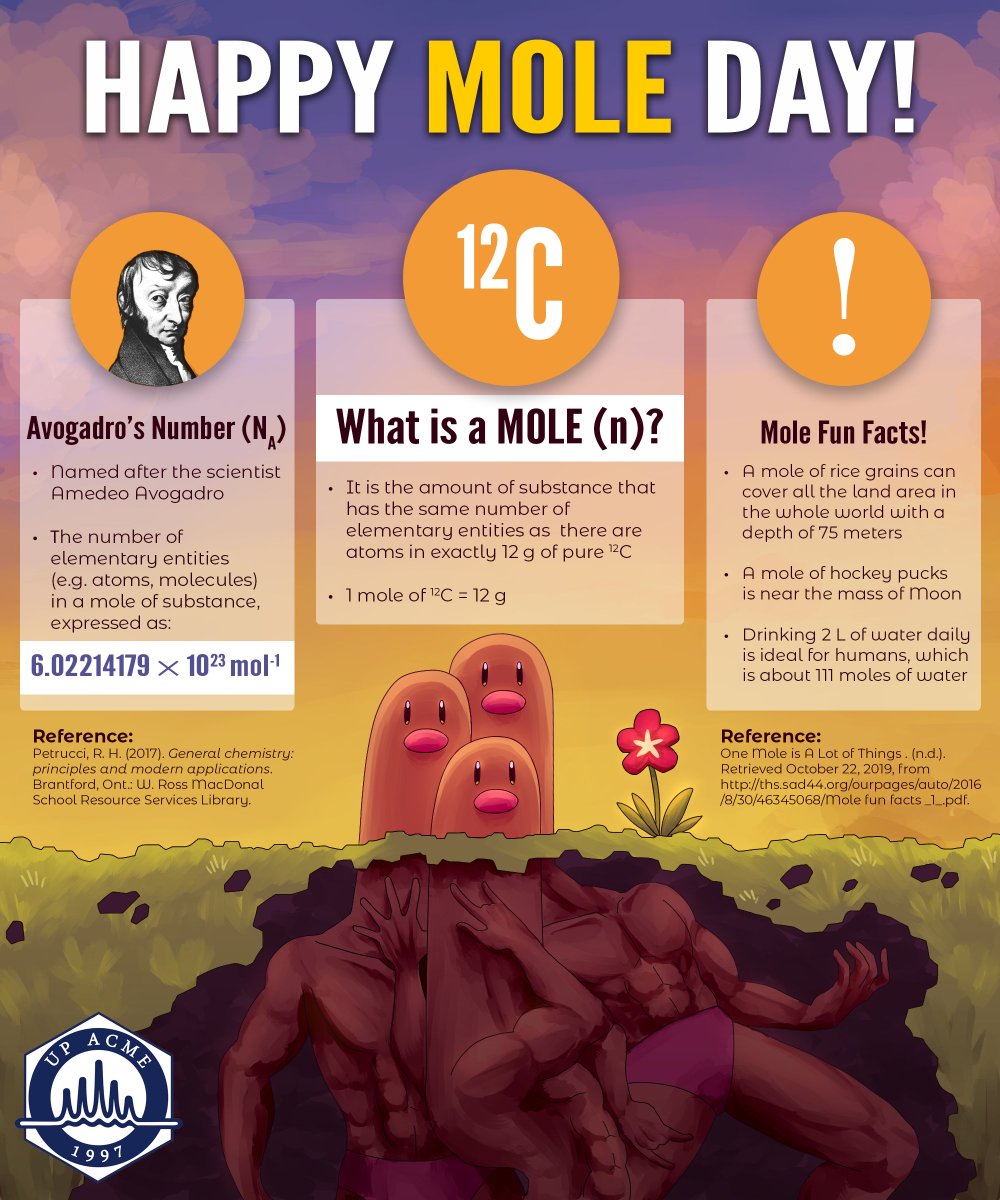
Mole is a word that those who have studied science may have heard at least once. Few people do not think that this is their mole or that they should call it a mole for some reason. The concept of mole was conceived by the scientist Amidio Avogadro. The concept of mole is based on the counting by weighing principle. This means that atoms and molecules are so small that tens of thousands of atoms can sit comfortably at the head of a needle. One gram of matter contains billions of atoms. These cannot be enumerated. That’s where the relevance of the mole concept comes into play.

A mole is a bridge between a mass and the number of atoms / molecules. Avogadro argued that the volume of a gas (that is, the area where the gas should be located) is related to the number of molecules or atoms it contains, and that even the nature of the gas is irrelevant. It was also found to be 22.414 liters in standard condition (zero degrees Celsius, one bar pressure). Similarly, in a mass equal to the atomic weight of the elements (although the atomic weight of each element is different), the number of atoms must be the same, Avogadro said. Joseph Lഷ്schmith later estimated that it was 6.02214076 × 10 ^ 23. (602214076000000000000000). According to the new definition, which came into force on May 20, 2019, the number of particles in a mole is exactly 6.02214076 × 10 ^ 23.

For example, if the atomic weight of hydrogen is taken together, one gram of hydrogen contains .06.02214076 × 10 ^ 23 atoms. At 12 grams of carbon, the same number of atoms are formed. This is because the atomic weight of carbon is twelve.

Similarly, if a mass equal to the molecular weight of a compound is measured, the contents will be the same number of molecules. The molecular weight of water is eighteen. Eighteen grams of water contains 6.02214076 × 10 ^ 23 water molecules. It was Avogadro who helped connect the concepts of atom and molecule. October 23 is celebrated as Mole Day every year, taking into account the fact that ten times twenty-three





Recent Comments