August 30, marked the 150th birthday of Ernst Rutherford, the founder of nuclear physics.

Ernst Rutherford was born in New Zealand on August 30, 1871, the son of farmer James Rutherford and Martha Thompson, and died in Cambridge on October 19, 1937. Wife: Mary Newton. Daughter: Eileen. Eileen’s husband is the famous physicist RH Fowler.
To the wonderful world of atomic physics
What awaited Rutherford at the Cavendish Laboratory in Cambridge was the awe-inspiring world of nuclear physics. The first research was on the ionization of gas molecules under JJ Thompson, the inventor of the electron. With Henry Becquerel’s discovery of natural radioactivity in 1896, Rutherford’s attention turned to the radioactivity of uranium. Thus, in 1898, alpha and beta rays were detected in radioactive radiation from uranium, and a few years later gamma rays were detected. A radioactive isotope of radon, formed by the depletion of thorium, was later discovered. Rutherford realized that a series of new elements would be formed by the decay of radioactive elements. It was an achievement that pointed to infinite possibilities in nuclear physics. Studies on the half-life of radioactive elements have also received much attention.

Looking for the proton
In 1886, Rutherford’s attention was drawn to the positively charged anode rays (canal beams) observed by a scientist named Goldstein during discharge tube experiments. He then filled a discharge tube with low-pressure air and observed the presence of hydrogen nuclei as alpha particles flowed through it. Recognizing that this was from the nitrogen atom in the air, he repeated the experiment by passing alpha particles through the nitrogen gas and observing the formation of more hydrogen nuclei. He realized that the hydrogen nucleus is part of every atom. This hydrogen nucleus was later called a proton.
Gold and alpha particles
Rutherford’s experiment with alpha scattering in 1911 led to some of the most significant discoveries in atomic history.
The high-energy alpha particles from the radium crashed into a thin layer of gold. The alpha particles passing through the gold plate were placed on a circularly arranged fluorescent zinc sulfide screen. Most of the alpha particles passed through the gold plate without any deviation. He observed that there were small changes in the path of some alpha particles and that very few alpha particles returned as if they had been hit by some small obstacle. He concluded that most of the volume of an atom is empty, and that all the positive charge and mass of the atom are concentrated in the nucleus.

Solar system model of the atom
The atomic model of the plum pudding model put forward by his mentor JJ Thompson did not seem at all acceptable to Rutherford. Rutherford proposed an atomic model known as the Solar System model based on his own inventions. According to this, as the planets orbit around the sun, the electrons travel in a circular path around the nucleus of the atom called the orbit. But according to James Clark Maxwell’s electromagnetic wave theory, a charged particle traveling in a curve will continue to emit radiation. In that case, the electrons orbiting the nucleus must continue to emit radioactive energy, narrow their orbits and eventually fall into the nucleus. But this does not happen in the atom. The Rutherford model failed to explain the stability of the atom.
Many of Rutherford’s discoveries, both direct and indirect, opened the door to new possibilities. Rutherford’s research was influenced by Niels Bohr’s atomic model, Mossouli’s experiment with the fingerprinting of elements, and Otto’s nuclear fission experiments.

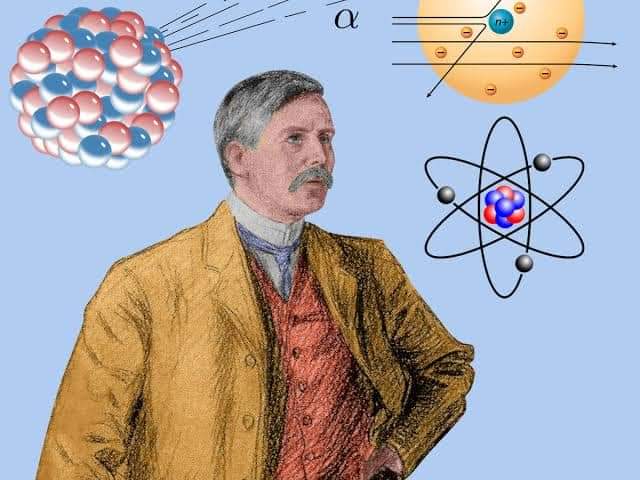



Recent Comments