
LPG is composed of propane (C3H8) and butane (C4H10), close to gasoline, and has twice the flammability of natural gas, so we can not see the flame completely by burning completely and well in the normal atmosphere,
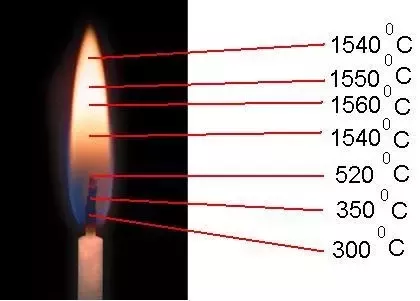
but the heat is biogas, methane (CH4), the same compound in natural gas, and carbon dioxide (CO2), and many other gases, as well as water vapor.
Pressure. ,, LPG pressure is high, the burner on the outside of the nozzle burns well as air in the mix, so the whole flame can not be seen,

the biogas pressure will not mix well with low, there will be many colors like an old kerosene lamp when burning will be a well visible flame





Recent Comments