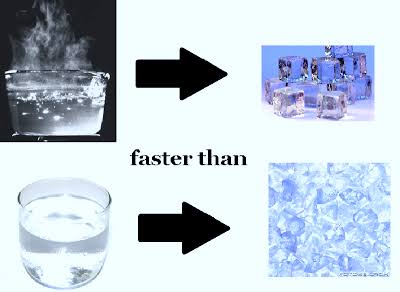
Erasto Empemba was a schoolboy in Tanzania in 1963.
While making ice cream with his friends, he noticed that hot milk freezes faster than cold milk.

Hence the name Empemba effect.
But this effect has been observed since the time of Aristotle. But chemists have struggled to give an accurate scientific explanation.
There is a reason for that.
Warm water will evaporate quickly. This will help it to freeze quickly.
But this explanation was not very convincing. Therefore, accurate detection is essential.
Meanwhile, in 2013, Xi Zang and his colleagues at Nanyang Technological University explained that this effect is caused by the strange behavior of water’s bond.
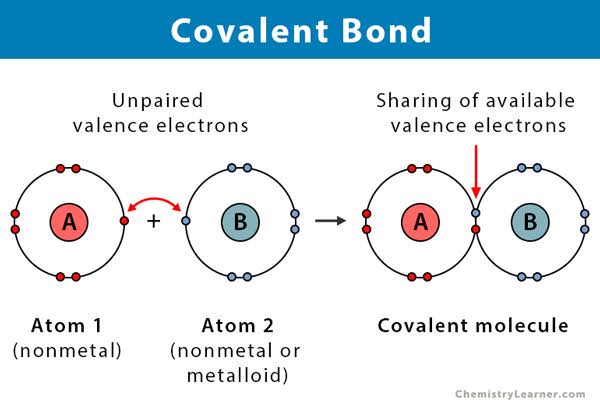
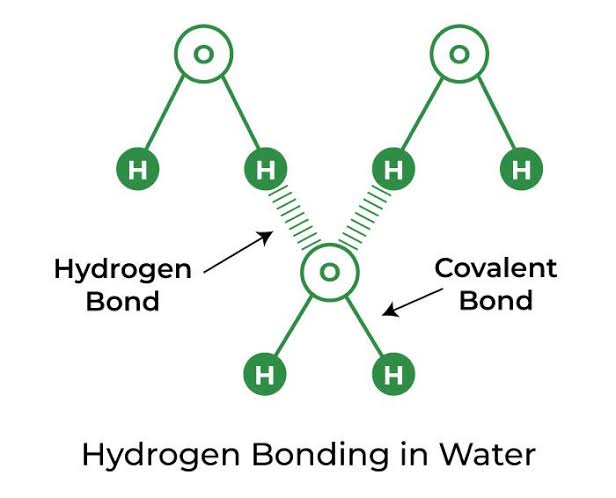
A water molecule is formed by covalent bonding of two hydrogen atoms and one oxygen atom.
★[ Covalent bond : share electrons with each other and form a bond. ]
In this way each water molecule is held together by Hydrogen Bonding.
★[ Hydrogen bond : Bond between Hydrogen and highly electro-ve atoms. It has intermolecular and intramolecular hydrogen bonds. It is intermolecular that holds water molecules together. ]
Molecules move very quickly in warm water. Therefore, the hydrogen bond between each water molecule will become very weak and the covalent bond will shrink.
Shrinking like this indicates that the bonding will be very strong.
Energy is stored in the shrink bond.
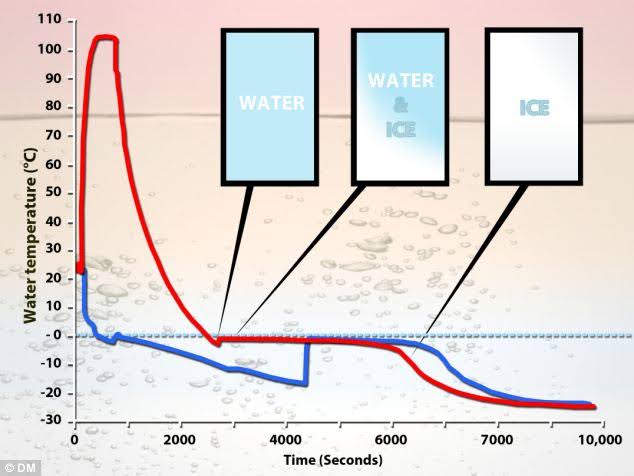
Now this warm water freezes.
Then the water molecules get closer together and the weak hydrogen bond becomes strong.
This is because the covalent bond within the water molecules becomes weak.

The previously stored energy is released by becoming weak.
It is equivalent to cooling.
So freezing speed increases as compared to cold water.





Recent Comments